Label-free and label-dependant isolation of Circulating Tumor Cells (CTCs) with microfluidic chips for liquid biopsy
Microfluidics has enabled miniaturized microdevices, often called microchips or biochips, to be fabricated for a wide variety of applications. The microfluidic chips have opened a new window of opportunity in various fields including fundamental biology, and chemistry research as well as clinical health and point-of-care applications. Among the health-related applications, circulating tumor cell (CTC) capturing using microfluidic chips can be of significance for diagnostic and liquid biopsy.
Circulating Tumor Cells (CTCs) are a subgroup of cancer cells that are detached from a primary tumor and shed into the bloodstream. A portion of the CTCs can seed in another anatomical site in the body leading to metastasis and death. The CTCs are surrogates of the associated tumor and thus bear invaluable information. CTCs can be used as a real-time liquid biopsy to give the clinician a good insight into the tumor’s condition omitting the need for invasive approaches. Early-stage cancer patients can benefit from early intervention and prognoses using CTCs. Additionally, the CTCs can get interrogated to draw the treatment-resistance profile of the tumor. They are usually present in the blood-stream at a very low concentration. Therefore, the blood sample first needs to be manipulated to enrich the CTCs population. Next, the CTCs can be characterized to extract the desired data.
CTCs isolation conventions and the role of microfluidics
Conventional methods for enrichment/isolation of CTCs either require expensive instrumentations or suffer from poor efficiencies. This imposes challenges for employing CTCs as a form of liquid biopsy. Some of the methods rely on epithelial markers that are unreliable due to the epithelial-mesenchymal transition of CTCs. Density centrifugation methods are also not highly accurate and lack high sensitivity. Microfluidics, however, has opened a window of opportunities for CTC research. Microfluidics fabrication processes allow handling of minuscule amount of fluids and showing great promise in cell sorting. Microfluidic chips have presented a bright outlook of the future of CTC isolation and characterization.
Microfluidic devices for isolating circulating tumor cells are either label-free or label-dependant. These label-free chips, which are often made of PDMS, rely on the intrinsic difference of the circulating tumor cells such as the size or density for isolating them. In label-dependent CTC isolation chips, the cells are first labeled using their epithelial markers and then captured based on the labeling method.
Label free microfluidics methods for circulating tumor cell capturing
Circulating tumor cells are much lower in numbers compared to the other cells in body fluids. In one mL of blood, 1-3000 CTCs might exist in a sea of a billion red blood cells and a few million white blood cells. This makes isolating them a challenge.
Label-free microfluidic techniques take advantage of the CTCs’ differences with other cells such as red blood cells and white blood cells to be able to isolate them and use them as liquid biopsy. Physical hallmarks of CTCs are:
– Often have a larger size than blood cells
– Higher density
– Stronger charge
Label-free isolation of the CTCs shares many things in common with microfluidic cell sorting. Label-free CTC isolation chips might use one or any of the abovementioned hallmarks for cell separation. These approaches include both active and passive methods. Biomarkers can be expensive and increase the costs of the experiments. Label-free methods have the advantage of being independent of biomarkers. Also, they are of import when the CTCs do not express surface markers strongly.
– Filtration: In filtration, topographical features of the microchannel filter the CTCs. Mechanical filtering in microfluidics includes microseiving, pillars, weirs, and cross-flow filtration.
– Hydrodynamics: Microfluidics is associated with specific flow features. In hydrodynamic techniques, the unique features of flows in micron scale such low Reynolds number to isolate the CTCs. These microfluidics techniques involve deterministic lateral displacement (DLD), inertial focusing, dean flow fractionation, pinched flow fractionation.
– Acoustophoresis: Acoustic waves can exert force on cells inside microfluidic channels. The amount of force that they cells experience is related to their properties. Microfluidics Acoustophoresis uses this concept to differentiate the cells and isolate the CTCs.
– Electrokinetics: Electric field and its interaction with the cells is the basis of CTC isolation here. Different cells in an electric field can behave differently. This different behavior can be tailored to isolate the CTCs. Electrophoresis and dielectrophoresis (DEP) are the subcategories that take different approaches.
More details about microfluidic cell sorting including active and passive methods can be found here.
Label-dependent microfluidics methods for circulating tumor cell capturing
Here, the epithelial surface markers of the circulating tumor cells can are used as biomarkers including EpCAM, EGFR, HER2, and MUC1.
– Immunofluorescence: These microfluidics biochips can be substituted for large and costly Fluorescence-activated cell sorting (FACS) machines. Here, the CTCs should be tagged with antibodies and fluorescent tags and sorted based on the fluorescent signal.
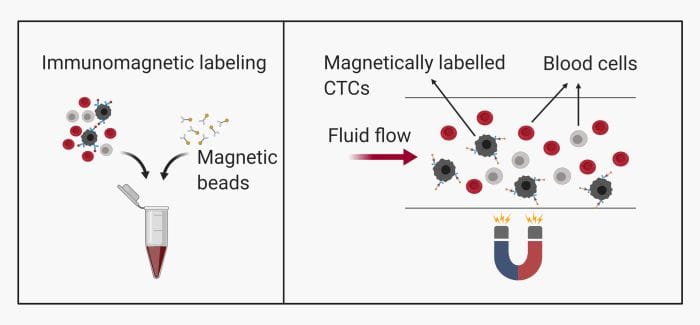
– Immunomagnetic: This method is common and includes labeling the CTCs with magnetic beads. They can then be pulled toward a specific zone on the microfluidics chip using magnets.
– Immunocapturing: As the name implies, one or multiple antibodies can be used to capture the CTCs. This microfluidics method requires a good mixing of the sample to ensure the CTCs have the chance to meet the antibodies. Various methods mixing methods such as meandering microchannels, nanomaterials, patterned surfaces, and microarrays can be helpful.

Microfluidics Characterization of CTCs
CTC enrichment is just the beginning. Upon enrichment, CTCs need to be characterized to identify the origin and extract genetic data. Common methods are PCR, ELISA, and cytometry. Isothermal and non-isothermal nucleic acid amplification methods such as PCR are easily integrable with microfluidic chips. Some approaches hinge upon amplifying specific mRNA in cell-free media while others require amplification after cell lysis. Depending on the technique, one or several microfluidic sample preparation chips might be needed. Recently, electrochemical readout techniques such as electrochemical impedance spectroscopy (EIS) have also gained attention.
Microfluidic approaches allow gentle isolation of live cells and thus enable many downstream analyses that rely on captured live CTCs.