Microfluidic technology for versatile fabrication of microfibers and microparticles
Microfluidic microfiber fabrication for tissue engineering applications
Micro/nanofibers have gained a lot of attention lately due to their extensive applications in tissue engineering and regenerative medicine. The advent of Microfluidics also revolutionized the techniques for generating microfibers allowing precise control over the morphology, material, size, and structural anisotropy as well as the biological application of these microfibers. A wide variety of microfluidics techniques for manufacturing a broad range of microfibers with various materials and morphologies have been developed to date.
Microfibers can either be used in the plain form for coating the substrates or scaffolding cell cultures or be filled with cells, medicine, and nanoparticles. Micro/nanofibers are excellent candidates for cell cultures scaffolds. They mimic the natural condition of the tissues in the body where fibrils are intertwined in a matrix. The latter type where nanoparticles are embedded in the microfiber is useful for the time-controlled release of nanomedicine.
How are the microfibers made using microfluidics?
Microfluidics technology creates extensive opportunities for custom-made fabrication of microfibers by running prepolymer and sheath flows through microfluidic channels.
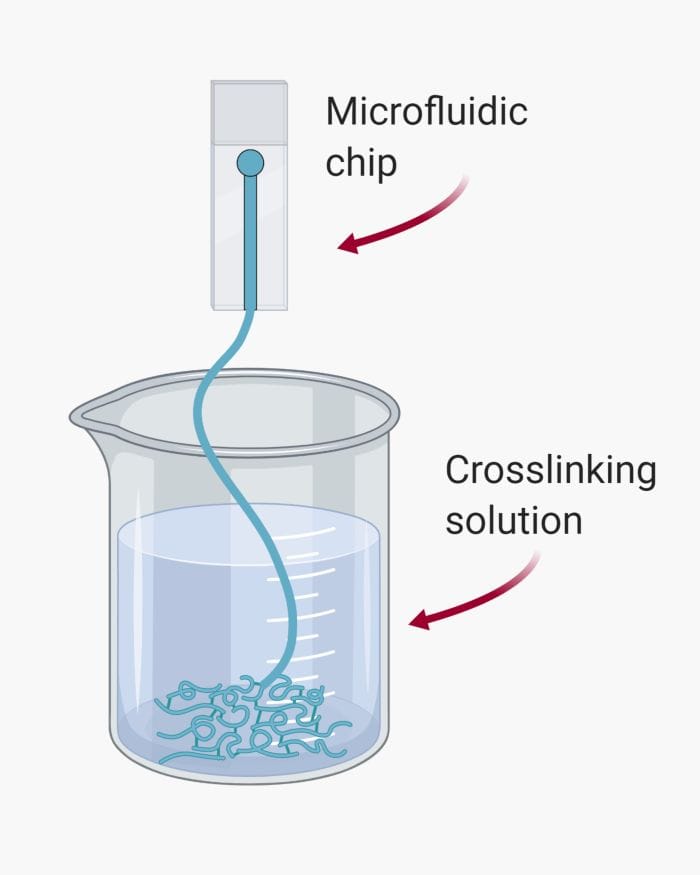 Microfluidics is associated with laminar flow. This feature allows two or multiple streams of liquids to flow alongside each other without mixing. This feature can be tailored for microfiber fabrication. For example, two streams of polymers with different properties can run next to each other followed by photo-crosslinking to fabricate a bi-material microfiber. By changing the arrangement of fluid flows, different microfibers can form.
Microfluidics is associated with laminar flow. This feature allows two or multiple streams of liquids to flow alongside each other without mixing. This feature can be tailored for microfiber fabrication. For example, two streams of polymers with different properties can run next to each other followed by photo-crosslinking to fabricate a bi-material microfiber. By changing the arrangement of fluid flows, different microfibers can form.
Crosslinking is an important step in microfiber production. Care should be taken so channels in the microfluidic device is not clogged while crosslinking the pre-polymer. The proper crosslinking method varies depending on the polymer type and the desired morphology for the microfiber. There are three common techniques for solidifying microfibers.
– Solvent extraction: The crosslinking occurs via an inversion of the phases. The sheath flow and polymer run through the microchannel and form a coaxial flow. The solvent in the polymer switches with the sheath fluid allowing the microfiber to solidify.
– Chemical crosslinking: A small molecule or ion causes the crosslinking in this method. The microfiber can be crosslinked to various degrees on the chip or be crosslinked off-chip.
– Photo-crosslinking: As the name implies, a photo-polymerizable polymer is needed here to crosslink the polymer. 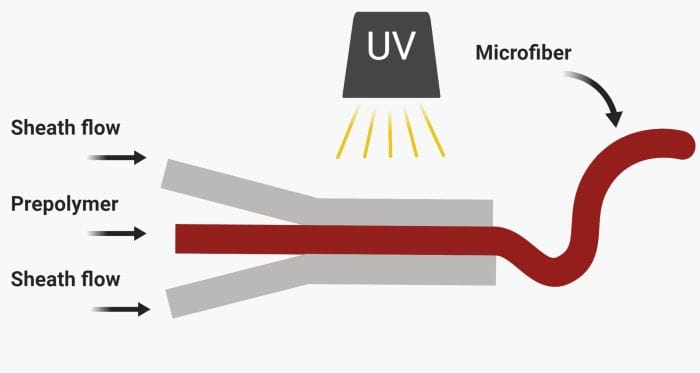
What type of microfibers can be made using microfluidics?
Microfluidics chips allow microfiber with uniform shape and size to be manufactured. The sizes vary from a few microns to hundreds of microns. Although most of the reported microfibers are in micron-scale, nanofibers are also achievable by modification in the input material. The microfluidics chips are also compatible with a wide range of materials. Besides, the chips allow unprecedented flexibility in altering the microfiber structure. Examples include but are not limited to:
– Hollow microfibers
– Microfibers with a different core material
– Multi-component microfibers
– Microfibers with a concentration gradient
Cell-laden microfibers are known to be building blocks for regenerative medicine and tissue reconstruction. Microfluidic chips are biocompatible and allow cell-laden microfibers to be made easily. Other than cells, nanoparticles, proteins or drugs can mix with the input material and get encapsulated within the microfiber for therapeutic purposes.
Microparticle synthesis production via microchips
When it comes to particles, two features are crucial: morphology and uniformity. Conventional approaches such as mechanical agitation are very poor in these regards. However, microfluidics provides a powerful tool for generating monosize particles of both spherical and custom shapes. Among microfluidic techniques, droplet microfluidics and photolithography have proven successful in particle fabrication. Droplet microfluidics is an excellent choice for spherical particles whereas the photolithography technique is suitable for custom-shaped microparticles.
Droplet microfluidics is the most popular method for particle generation. It allows very high particle generation frequencies in the order of kHz. The channels can be parallelized to increase the production rate. In this technique, a stream of liquid breaks into droplets by another stream to form polymeric droplets. Next, the droplets undergo a chemical reaction to solidify and form microparticles. Since the final solidified product originates from spherical droplets, the particles’ shape will always be spherical. The particles are highly uniform and mono-size with this approach. The input flow rates play an important role in determining the final particle size. The microfluidic chips are fabricated to operate over a span of flow-rates leading to generating particles with different sizes. Other factors that affect the droplet size are fluid viscosity and interfacial tension.
How to make spherical microparticles using microfluidics?
Spherical microparticles are the most common form of microparticles formed using microfluidic chips. All you need to generate spherical microparticles are the followings:
– Droplet generator chip
– A carrier phase and a polymeric solution: The carrier phase breaks the polymer solution into droplets. The droplets can then polymerize to
– Fluid delivery system: Syringe pumps are the most popular form of pumps used in microfluidics. You can easily control the input flow rates of both phases to ensure uniform droplets. You can also change the droplet size by adjusting the flow rates and flow rate ratios.
Depending on the polymeric solution used, the solidifying method can differ. Common methods include:
– Thermal polymerization where droplets undergo a temperature change to solidify
– Photopolymerization where the droplets experience UV exposure to polymerize
– Chemical polymerization where a third solution mixes with the droplets to solidify them
Droplet microfluidics also allows a bit of flexibility in the particle shape. Droplets adopt an elliptical shape if they pass through a channel less wide than their diameter. Polymerization can occur when passing through this microfluidics channel to form elliptical particles.
Core-shell particles, also known as double emulsions, are another type of spherical particles formed by microfluidic droplet generators. Core-shell particles are a great option for drug delivery applications. The core can include the drug while the shell can deteriorate over time for timed release of the core drug. The microfluidics chips used for creating core-shell droplets are called double emulsion chips. The double emulsions form in two steps. First, the inner core forms in the first droplet generator junction. Next, the inner droplet moves to a second junction where it emulsifies and makes the outer shell. Depending on the core, shell, and the outer carrier phase, the droplets are either water in oil in water (W/O/W) or oil in water in oil (O/W/O).
How can custom-shaped microparticles be made using microfluidic chips?
Custom-shaped microparticles have gained a lot of attention lately. This method is capable of generating microparticles of desired shapes and structures. As opposed to spherical particles, here there is no need for a droplet generator chip. Instead, a photomask constituting the desired shape should be used. The photomask lies on a straight channel where a photo-crosslinkable material is passing. Next, a UV lamp on top of the photomask turns on that passes through the printed shapes on the photomask and crosslinks the material underneath. The custom-shaped microparticles then flush out of the chip. The production can have a continuous or a flow-stop-flow pattern depending on the crosslinking rate. Multiple microfluidics streams can flow alongside each other to have microparticles with heterogeneous structures.


